SS 620:2016 医疗器械良好分销规范
SS 620:2016 医疗器械良好分销规范
The storage, handling and distribution of medical devices can be carried out by different organizations. Organizations are required to monitor of the storage and distribution activities of medical devices to prevent mix-ups and contamination, which in turn affect the quality and safety performance of medical devices.
The objective of SS 620 Good Distribution Practice for Medical Devices is to ensure the quality and integrity of the medical devices throughout the distribution process, to enhance the confidence level and safeguarding the welfare of consumers.
What is SS 620 Certification?
SS 620 Good Distribution Practice for Medical Devices- Requirements was developed by SPRING Singapore under the Singapore Standardisation Programme.
SS 620 Good Distribution Practice for Medical Devices focuses mainly on the requirements for distributors and importers to ensure safety, quality, and performance of medical devices throughout the import and distribution related activities for all medical devices in Singapore.
The design and implementation of SS 620 are varying across the size and structure of organization, the processes employed, and type of medical devices deals with. SS 620 focuses on criteria includes management responsibility, people involvement, premises and facilities, delivery process, installation and servicing process, good assembly and packaging practices, outsource activities, complaint handling, reporting for counterfeit, adulterated, unwholesome or tampered medical devices, field safety corrective action to regulatory authority.
With SS 620, protection, cost efficiency and confidence are passed along to the consumers through high quality care. In overall, compliance with regulations and requirements benefits everyone and the final result is countless saved lives.
Organisations who are involved in wholesale and or importation of medical devices in Singapore are mandatory to have GDPMDS certified before applying for an Importer or Wholesale Licence. With effective from 9 November 2020, certification to GDPMDS based on Singapore Standard GDPMDS will be the only acceptable option.
过程方法
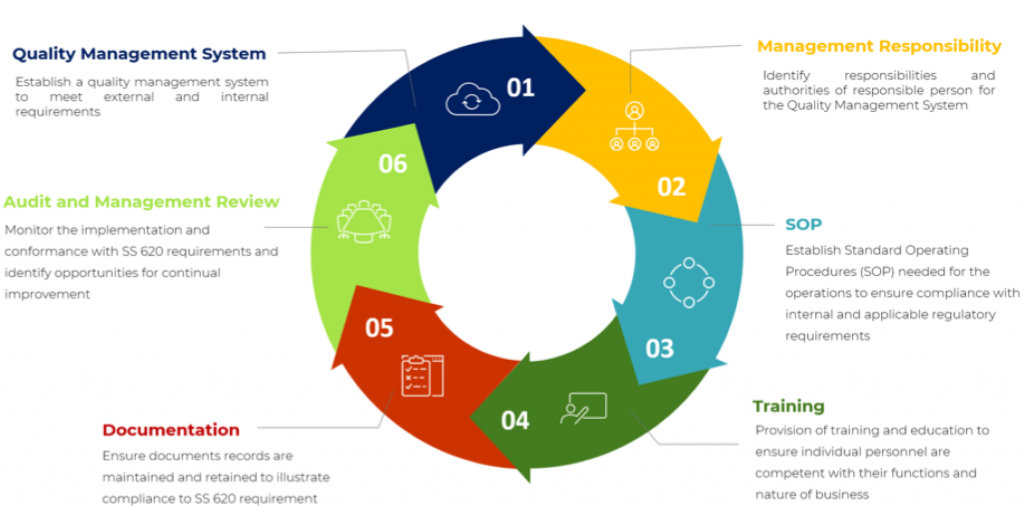
Why is SS 620 certification important?
In Singapore, importers and wholesalers dealing with medical devices should have Quality Management System that conforms with the Good Distribution Practice for Medical Devices (GDPMDS) to maintain the devices safety, quality and performance throughout the distribution process.
SS620 Certification Process
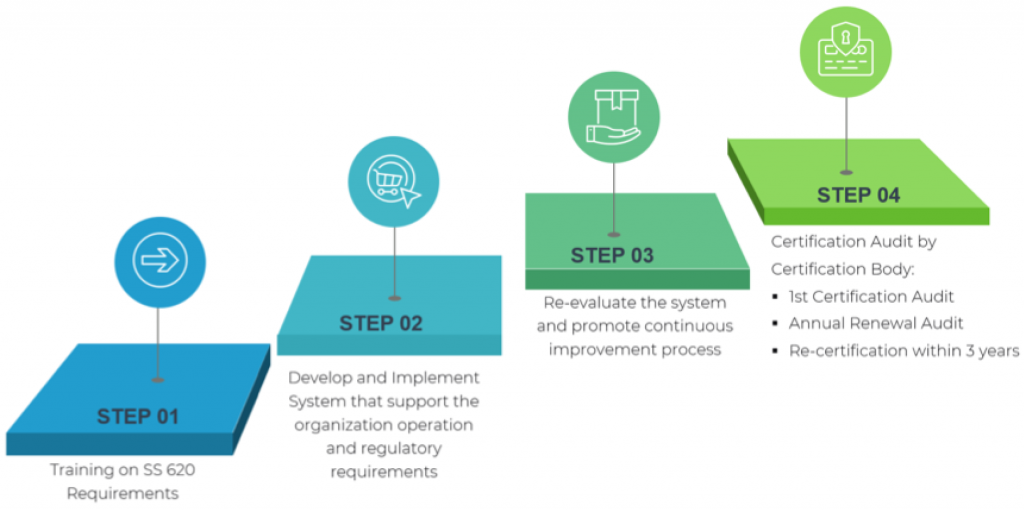
Step 1:
People and System Development
Step 1:
People and System Development
Training on SS 620 requirements.
步骤 2:
系统开发与实施
步骤 2:
系统开发与实施
Establish a system that encompass the processes or procedures that support the organization to meet the SS 620 requirements.
步骤 3:
内部审计和管理审查
步骤 3:
内部审计和管理审查
Conduct internal audit/review to constantly re-evaluate the system and promote continuous improvement process.
第 4 阶段:
认证审核
第 4 阶段:
认证审核
认证审核员进行认证审核(文件审查和实际应用评估)
Conferral of SS 620 Certificate
年度监督审计
3 年内重新认证
Training for SS 620
- SS 620:2016 Awareness Training
- SS 620:2016 Awareness Training
- SS 620:2016 Development and Implementation Training
- SS 620:2016 Development and Implementation Training
- SS 620:2016 Internal Auditor Training
- SS 620:2016 Internal Auditor Training
ISO 顾问是做什么的?
Our consultants team have vast experience partnering with organizations of all sizes and from all sectors and can provide the resources you need for successful ISO 13485 certification.
To establish effective management system in your company, our ISO Consultant consider your specific requirements and perspectives and guide your through the process for certification of your Quality Management System in accordance with SS 620 following these steps:
Step 1:
Site visit to understand your business operations, special storage, and handling conditions and categories of medical devices.
Site visit to understand your business operations, special storage, and handling conditions and categories of medical devices.
Step 2:
Site consultation & training for development of staff capability on quality management.
Site consultation & training for development of staff capability on quality management.
Step 3:
Establish of System Operational Procedures and Records.
Establish of System Operational Procedures and Records.
Step 4:
Advise on implementation process and analysis to improve Quality Management System.
Advise on implementation process and analysis to improve Quality Management System.
步骤 5:
支持各部门实施质量管理体系。
支持各部门实施质量管理体系。
步骤 6:
进行认证前审核,以验证贵组织是否做好了认证准备。
进行认证前审核,以验证贵组织是否做好了认证准备。
第 7 步:
在认证期间和认证后提供支持,以关闭任何审计发现,确保顺利和成功地进行认证。
在认证期间和认证后提供支持,以关闭任何审计发现,确保顺利和成功地进行认证。








