WSQ SS 620: 2016 (2021)
Good Distribution Practice For Medical Devices (GDPMDS) Internal Auditor Course
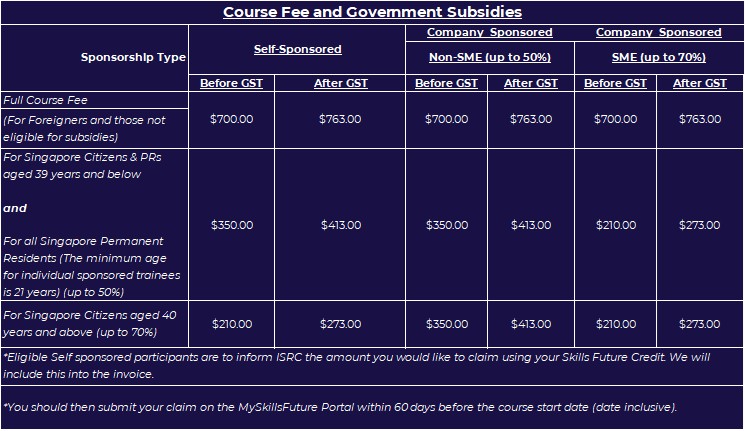
PRICE
SGD$700 [Subject to GST](SDF Grant Eligible)
DURATION
2 Days
LANGUAGE
English
SGD$700 [Subject to GST](SDF Grant Eligible)
2 Days
English
WHO SHOULD ATTEND
Quality Manager, SS 620:2016 Appointed Internal Auditor, Individual responsible for establishing, implementing, maintaining SS 620:2016
Quality Manager, SS 620:2016 Appointed Internal Auditor, Individual responsible for establishing, implementing, maintaining SS 620:2016
TSC COURSE NAME
Quality Assurance Management-4
TSC CODE
WST-QUA-4013-1.1
Quality Assurance Management-4
WST-QUA-4013-1.1
This course provides a comprehensive understanding of the SS620:2016 standard, which outlines the Good Distribution Practice for Medical Devices in Singapore.
The training equips learners with the knowledge and practical skills necessary to ensure medical devices are consistently stored, transported, and handled in a manner that maintains their quality and integrity throughout the distribution chain.