WSQ SS 620: 2016 (2021)
Good Distribution Practice For Medical Devices (GDPMDS) Internal Auditor Course
PRICE
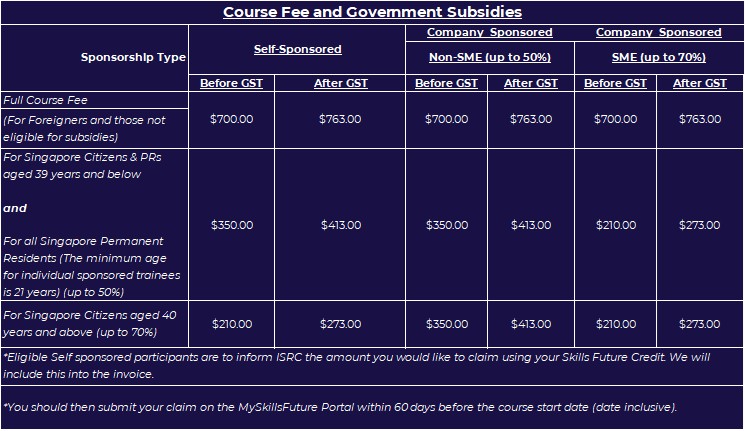
SGD$700 [Subject to GST](SDF Grant Eligible)
DURATION
2 Days
LANGUAGE
English
SGD$700 [Subject to GST](SDF Grant Eligible)
2 Days
English
WHO SHOULD ATTEND
Quality Manager, SS 620:2016 Appointed Internal Auditor, Individual responsible for establishing, implementing, maintaining SS 620:2016
Quality Manager, SS 620:2016 Appointed Internal Auditor, Individual responsible for establishing, implementing, maintaining SS 620:2016
TSC COURSE NAME
Quality Assurance Management-4
TSC CODE
WST-QUA-4013-1.1
Quality Assurance Management-4
WST-QUA-4013-1.1
This course provides a comprehensive understanding of the SS620:2016 standard, which outlines the Good Distribution Practice for Medical Devices in Singapore.
The training equips learners with the knowledge and practical skills necessary to ensure medical devices are consistently stored, transported, and handled in a manner that maintains their quality and integrity throughout the distribution chain.
Downloadable Resources
Course Content
The syllabus focuses on:
- Regulatory and Industry Landscape
- GDPMDS Quality Management System
- Roles and Responsibility of QA Monitoring
- Storage and Distribution Practices
- Regulatory and Industry Landscape
- GDPMDS Quality Management System
- Roles and Responsibility of QA Monitoring
- Storage and Distribution Practices
Pre-requisites*
-
Able to listen, read and write simple English
Mode of Training*
Physical Class
Physical class will be held at our training room within our office @ Lifelong Learning Institute
*A laptop is needed for this mode of training. Training Materials will be given in soft copy and emailed to all participants 1 working day before the course.
Assessment & Certification
- Part 1: Written Assessment (2hrs)
- Part 2: Individual Written Assessment Project (1 hr)Statement of Attainment (SOA) will be issued to participant who attains 100% attendance and COMPETENT for the assessments.
Learning Outcome
Upon completion of this course, participants will be able to:
- Understand the scope, principles, and requirements of SS620:2016
- Identify roles and responsibilities in the medical device distribution supply chain
- Implement and maintain a GDPMDS-compliant quality management system
- Prepare for audits and inspections by regulatory authorities (e.g., HSA)
- Assess and mitigate risks in the medical device distribution process
Need Any Assistance?
For more specific information on the course details and schedule, do get in touch with us. We are also able to customise a course for your company.
WSQ Cold Chain Management for Medical Goods
PRICE
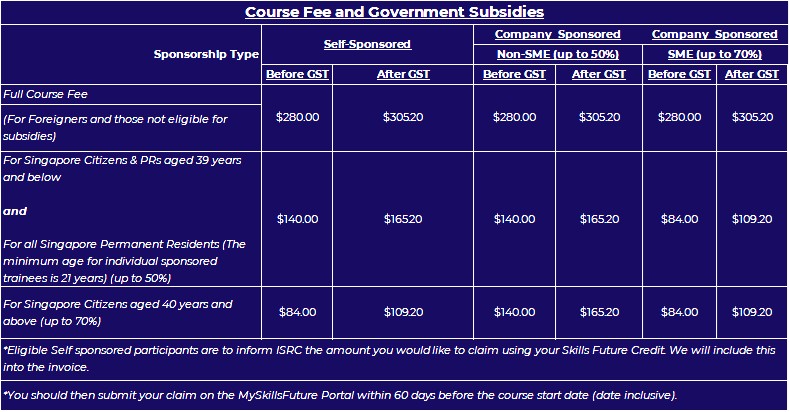
SGD$280.00 [Subject to GST](SDF Grant Eligible)
DURATION
1 Days
LANGUAGE
English
SGD$280.00 [Subject to GST](SDF Grant Eligible)
1 Days
English
WHO SHOULD ATTEND
- Logistics and supply chain professionals handling medical goods
- Pharmaceutical and medical device QA/QC personnel
- Warehouse and distribution managers / Supervisors
- Regulatory affairs and compliance officers
- Healthcare procurement and operations
TSC COURSE NAME
Cold Chain Operations Administration-4
TSC Code
LOG-FFO-4004-1.1
Cold Chain Operations Administration-4
LOG-FFO-4004-1.1
This course equips learners with comprehensive knowledge and practical skills for managing the cold chain of medical products including pharmaceuticals, vaccines, and biologicals. It covers regulatory requirements, cold chain equipment and packaging, monitoring systems, risk management, and audit readiness.
Course Fee and Government Subsidies
Downloadable Resources
Course Content
- Cold Chain Fundamentals
- Regulatory and Industry Standards
- Storage and Inventory Control
- Equipment, Packaging and Transport Methods
- Excursion Handling and Risk Mitigation
- Qualification, Validation and Audit Readiness
Pre-Requisites
-
Able to listen, read and write simple English
-
Able to listen, read and write simple English
Method of Study
Face to Face Classroom based training with practical case studies and group activities.
Assessment & Certification
- Part 1: Written Assessment (60min)
- Part 2: Individual Written Assessment Project (60min)
Statement of Attainment (SOA) will be issued to participant who attains 100% attendance and COMPETENT for the assessments.
Learning Outcomes
Upon completion of this course, participants will be able to
- Understand the principles and importance of cold chain management
- Identify temperature-sensitive medical goods and regulatory storage requirements
- Operate cold chain equipment and select appropriate packaging solutions
- Apply risk management and CAPA in cold chain disruptions
- Develop and evaluate cold chain monitoring and validation procedures
- Prepare for audits and regulatory inspections related to cold chain systems
Need Any Assistance?
For more specific information on the course details and schedule, do get in touch with us. We are also able to customise a course for your company.
WSQ Cold Chain Management for Medical Goods
PRICE
SGD$280.00 [Subject to GST](SDF Grant Eligible)
DURATION
1 Days
LANGUAGE
English
SGD$280.00 [Subject to GST](SDF Grant Eligible)
1 Days
English
WHO SHOULD ATTEND
- Logistics and supply chain professionals handling medical goods
- Pharmaceutical and medical device QA/QC personnel
- Warehouse and distribution managers / Supervisors
- Regulatory affairs and compliance officers
- Healthcare procurement and operations
TSC COURSE NAME
Cold Chain Operations Administration-4
TSC Code
LOG-FFO-4004-1.1
Cold Chain Operations Administration-4
LOG-FFO-4004-1.1
This course equips learners with comprehensive knowledge and practical skills for managing the cold chain of medical products including pharmaceuticals, vaccines, and biologicals. It covers regulatory requirements, cold chain equipment and packaging, monitoring systems, risk management, and audit readiness.
Course Fee and Government Subsidies

Downloadable Resources
Course Content
- Cold Chain Fundamentals
- Regulatory and Industry Standards
- Storage and Inventory Control
- Equipment, Packaging and Transport Methods
- Excursion Handling and Risk Mitigation
- Qualification, Validation and Audit Readiness
Pre-Requisites
-
Able to listen, read and write simple English
-
Able to listen, read and write simple English






