ISO/IEC 17025:2017 General Requirements for the Competence of Testing and Calibration Laboratories
ISO/IEC 17025:2017 General Requirements for the Competence of Testing and Calibration Laboratories
Meeting clients’ needs is essential in today’s business. ISO/IEC 17025:2017 enables an organization to meet the requirement for the competence of testing and calibration laboratories. The accreditation are for both locally and internationally and that brings out the confidence in your organization’s ability to to operate the laboratory competently and generate valid results.
What is ISO/IEC 17025:2017 certification?
ISO/IEC 17025:2017 is developed with the objective of promoting confidence in the operation of laboratories. The standards consist the requirements to fulfilled in order to shown that the laboratories are able to operate competently and produce valid results.
The use of ISO/IEC 17025:2017 will facilitate cooperation between laboratories and other bodies, and assist in the exchange of information and experience, and in the harmonization of standards and procedures. The acceptance of results between countries is facilitated if laboratories conform to the standards.
The following list are the fields that commonly obtained ISO 17025 Accreditation:
- Calibration & Measurement
- Chemical & Biological
- Civil Engineering
- Electrical Testing
- Environmental Testing
- Mechanical Testing
- Medical Imaging
- Medical Testing
- Non-Destructive Testing
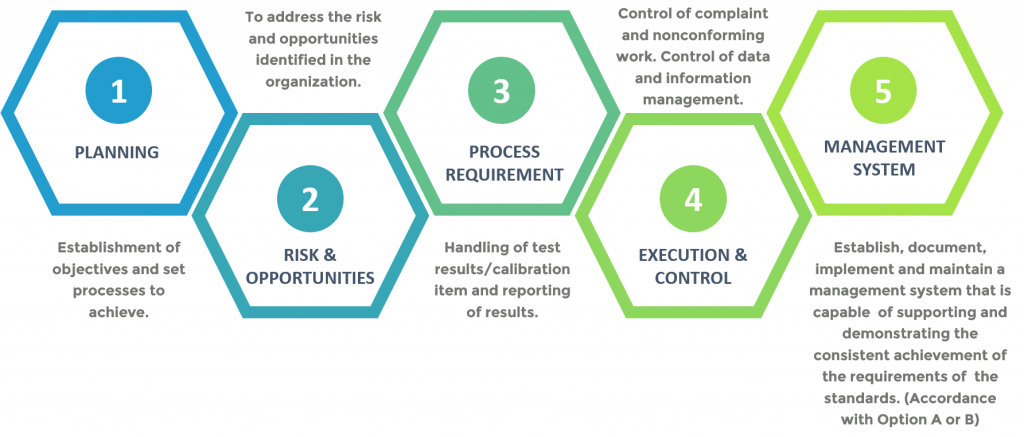
ISO/IEC 17025:2017 requires the laboratory to plan and implement actions to address risks and opportunities. The standards will address both risks and opportunities establishes a basis for increasing the effectiveness of the management system, attain improved results and preventing negative effects. The laboratory is responsible to decide which risks and opportunities need to be addressed.
Laboratories that conform to ISO/IEC 17025:2017 will also operate generally in accordance with the ISO 9001’s principles.
Process Approach
Why is ISO 17025:2017 certification important?
Organizations certified in ISO 17025 show evidence of the ability to consistently provide accurate testing and calibration that meet customer and regulatory requirements. Organization have the competitive edge on their analysis results on the testing and calibration method as ISO 17025 certification brings along with customer’s confidence over the test and calibration results.
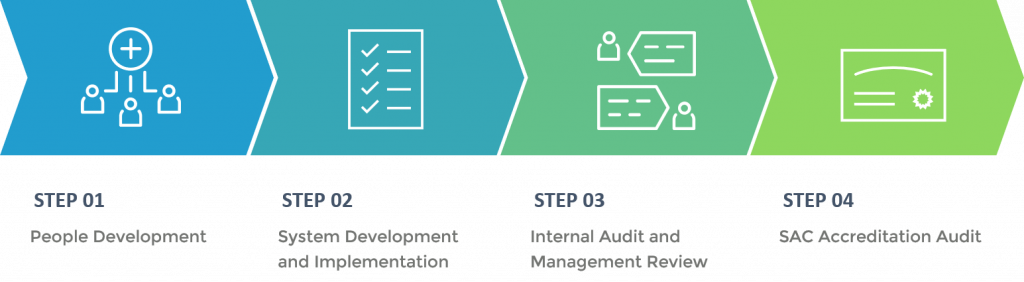
ISO 17025:2017 Accreditation Process
Step 1:
People Development
Step 1:
People Development
Training on ISO 17025 requirements.
Step 2:
System Development and Implementation
Step 2:
System Development and Implementation
Establish a Laboratory Management System that encompass the processes or procedures that support the organization to meet the ISO 17025 requirements as well as the client’s expectations.
Step 3:
Internal Audit and Management Review
Step 3:
Internal Audit and Management Review
Assist client when they conduct internal audits/reviews to constantly re-evaluate the system and promote a continuous improvement process.
Stage 4:
SAC Accreditation Audit
Stage 4:
SAC Accreditation Audit
SAC Accreditation auditor conduct accreditation audit (document review and practical application assessment)
Conferral of Accreditation Certificate
Annual Surveillance Assessment based on 4-year accreditation cycle
Re-assessment prior to expiry of accreditation certificate
Training for ISO 17025
- ISO 17025:2017 LMS Awareness Training
- ISO 17025:2017 LMS Awareness Training
- ISO 17025:2017 LMS Development and Implementation Training
- ISO 17025:2017 LMS Development and Implementation Training
What does an ISO consultant do?
Our consultants team have vast experience partnering with organizations of all sizes and from all sectors and can provide the resources you need for successful ISO 17025 accreditation.
To establish effective laboratory management in your company, our ISO Consultant considers your specific requirements and perspectives and guide your through the process for accreditation of your Laboratory Management System, following these steps:
Step 1:
Site visit to understand your business operations and key risk.
Site visit to understand your business operations and key risk.
Step 2:
Site consultation & training for the development of staff capability on laboratory management.
Site consultation & training for the development of staff capability on laboratory management.
Step 3:
Writing of Laboratory Management Process and Procedures.
Writing of Laboratory Management Process and Procedures.
Step 4:
Advice on Key Performance Indicators (KPI’s) and SAC measurement traceability of analysis results.
Advice on Key Performance Indicators (KPI’s) and SAC measurement traceability of analysis results.
Step 5:
Support individual departments on their implementation of Laboratory Management System.
Support individual departments on their implementation of Laboratory Management System.
Step 6:
Assist in pre-accreditation audit to verify readiness of your organization for certification.
Assist in pre-accreditation audit to verify readiness of your organization for certification.
Step 7:
Support during accreditation and post-accreditation to close-up any audit findings to ensure smooth and successful accreditation.
Support during accreditation and post-accreditation to close-up any audit findings to ensure smooth and successful accreditation.






